THESIS
Draft thesis plan:
OUTCOMES OF MONITORING ACUTE AND CHRONIC GLOMERULAR INJURIES WITH SPOT AND 24 HOUR URINARY PROTEIN CREATININE RATIO ESTIMATION:
PROBLEM STATEMENT / BACKGROUND
"In most patients with evidence of glomerular disease there is no single measure that provides a specific diagnosis, not even kidney biopsy. To achieve a specific diagnosis, and all that this implies for appropriate management, it is often necessary to test broadly and use a systematic approach."
However in many patients the glomerular disease may progress and an important factor that is linked to patient survival is residual renal function, clinically assessed as the amount of daily urinary output. Many factors conspire against this important variable: aging, the etiologies of renal failure and time on dialysis.
AIM :
To study outcomes of monitoring acute and chronic glomerular injuries with random and 24 hour urinary protein creatinine estimation.
OBJECTIVES
1.To assess spot urine protein creatine ratio.
2.To assess 24hr urine protein, and various clinical and investigational characteristics of patients with glomerular injury .
3.To analyse patient outcomes based on mortality , need for dialysis, cardio vascular diseases(myocardial ischaemia), Cerebrovascular disease (stroke) and Complete or Partial Remission of proteinuria and hypothyroidism
PATIENTS AND METHODS:
PLACE OF STUDY: Department of General medicine , Kamineni Institute of Medical Sciences, Narketpally.
STUDY PERIOD: November 2022- October 2024
STUDY DESIGN : Non experimental (Observational) qualitative Prospective Study
SAMPLE SIZE: 50 patients
INCLUSION CRITERIA:
• Proteinuria patients of any gender above or equal to 18yrs of age at the time of presentation.
• Patients presenting with a combination of proteinuria and complex etiological events and outcomes.
EXCLUSION CRITERIA:
1.Patients below 18 yrs of age (minors)
2.Patients not capable of giving consent (mentally-ill patients)
3.Patients not willing to participate in study (non-consenting patients)
METHODOLOGY
Patients having proteinuria (CUE disptick showing ≥+1 albuminuria )were selected from general medicine opd or casuality .
Consent from the patient is taken.
Patient is examined and detailed history taken.
spot urine protein creatinine ratio was estimated and noted.
24 hour urine protein was estimated and noted.
Then each patient was followed after every 6months till the end of thesis study period, Spot UPCR and 24 hour urine protein estimations noted for every followup.
Collection of 24hr urine sample:On admission each consenting patient was taught how to collect 24-hour urine for determination of proteinuria. The patient was asked to void at 6.00am and then collect all the urine subsequently until 6.00am the next morning. A large clean, dry and wide-mouthed plastic container was provided for each patient to collect the urine which was poured into a larger container until the collection was complete. The total volume was noted and the urine was stirred to ensure homogeneity. Then large container bearing her name,age, hospital number was sent immediately to the laboratory for processing.
Collection of spot urine sample:
each consenting patient was instructed adequately on how to collect clean catch midstream urine with the next urge to urinate.The first part of the urine was let out and about 10 ml of the midstream urine was collected into the sterile universal bottles which bore name,age,sex and hospital number. The urine samples in the universal bottle were transported to the laboratory for processing with in one hour,and where immediate processing was not possible, the samples were also promptly refrigerated at 4°C.
The urine for 24 hours protein and spot protein concentration was estimated by using turbidimetric method in auto analyzer (Mindray BS390) .
24 hours urine creatinine and Spot Urine Creatinine was estimated by using modified Jaffe’s method in auto analyzer (Mindray BS390).
Glomerular filtration rate(GFR)was calculatedfromageand plasma creatinine byModified Diet in Renal Disease (MDRDFormula.
GFR(mL/min/1.73m2)=186x(Pcr)1.154x(age)0.203x(0.742if female)x(1.210ifAfrican American)
The GFR is expressed in mL/min/1.73m2
PROFORMA (data to be captured)
Demographics
Patient event data reflected in a narrative history of the sequence of events leading to the current presentation and outcomes
Body data from clinical general and systemic examination
Pallor
Lymphadenpathy
Nails
Organomegaly
Body data from laboratory Investigation :
CBP with peripheral smear
Urine for cue
Urine for random protein creatinine ratio
Urine for 24 hour urine protein and creatinine and electrolytes where indicated
Iron profile with serum ferritin
Serum albumin
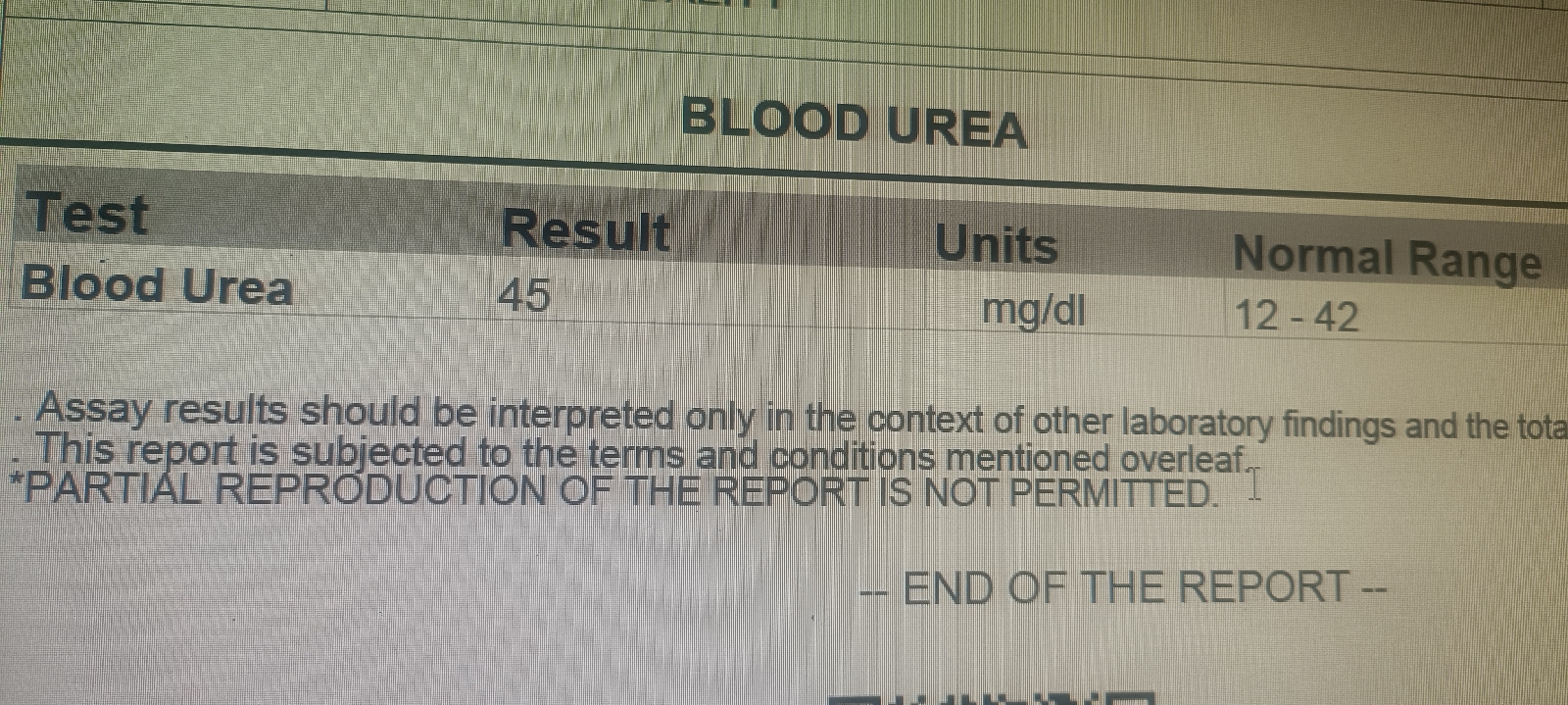
Serum urea and creatinine, RFT
Special tests on indication :
Thyroid function tests
ECG
2D ECHO
Renal biopsy if indicated
Data from Patient reported outcomes :
REFERENCES:
Quoted from :
1) Cravedi P, Remuzzi G. Pathophysiology of proteinuria and its value as an outcome measure in chronic kidney disease. Br J Clin Pharmacol. (2013) 76:516–23. 10.1111/bcp.12104
2) Trimarchi, H. (2013). Remnant Proteinuria in Chronic Hemodialysis. In (Ed.), Hemodialysis. IntechOpen. https://doi.org/10.5772/53657
Full text : https://www.intechopen.com/chapters/40473
3)Amin SV ,Illipilla S, Hebbar S , et al.Efficacy of spot protein creatinine
ratio and dipstick for prediction of proteinuria.Int J of Hypertens
2014;2014/941408.
Shazia Majid Khan et al Comparison of spot protein creatinine ratio vs
24 hours urinary protein in pre eclampsia. Int J of Gynecol & Obstet
Research2014;2:67-72.
4) Revankar M,Divakar NR et al. A correlative study of 24 hr urinary
protein and random urinary protein creatinine ratio.Int J of Pharm&
Biomed Research2013;4(2):93-96.
5)Eigbefoh J ,Abebe J , Odike M, Isabu D et al.Random protein creatinine
ratio for quantificationof proteinuria.The Internet J of Gynecol &
Obstet2006;8(1).
LINKS TO PATIENT E Log:
http://kattaphanikeerthanarollno72.blogspot.com/2022/08/a-46-year-old-male-with-pedal-edema-and.html